Monoclonal antibody treatment of Multiple Myeloma | Leaders in Pharmaceutical Business Intelligence (LPBI) Group
![Calcium phosphate engineered photosynthetic microalgae to combat hypoxic-tumor by in-situ modulating hypoxia and cascade radio-phototherapy [Abstract] Calcium phosphate engineered photosynthetic microalgae to combat hypoxic-tumor by in-situ modulating hypoxia and cascade radio-phototherapy [Abstract]](https://www.thno.org/v11/p3580/toc.jpg)
Calcium phosphate engineered photosynthetic microalgae to combat hypoxic-tumor by in-situ modulating hypoxia and cascade radio-phototherapy [Abstract]

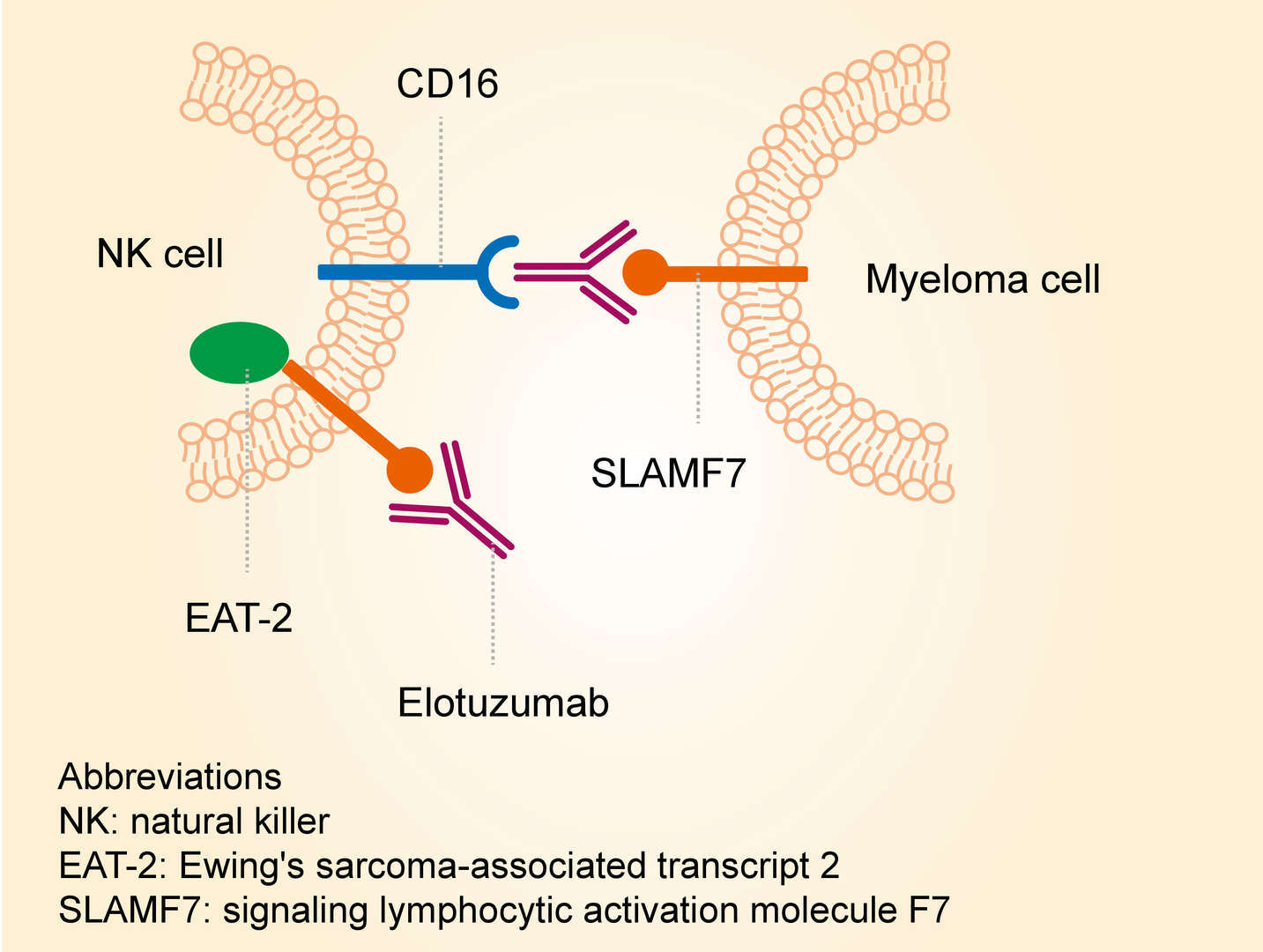
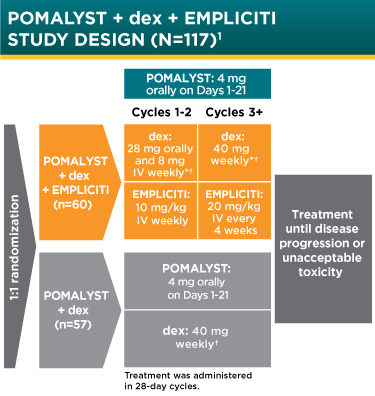
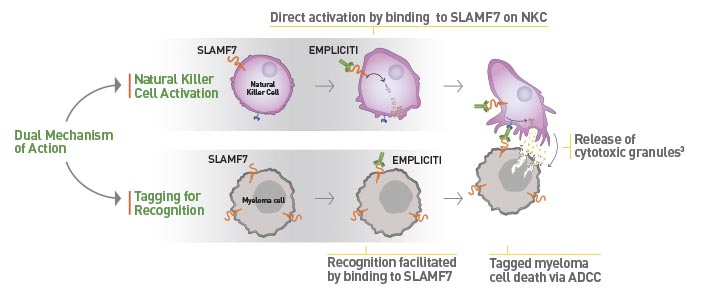
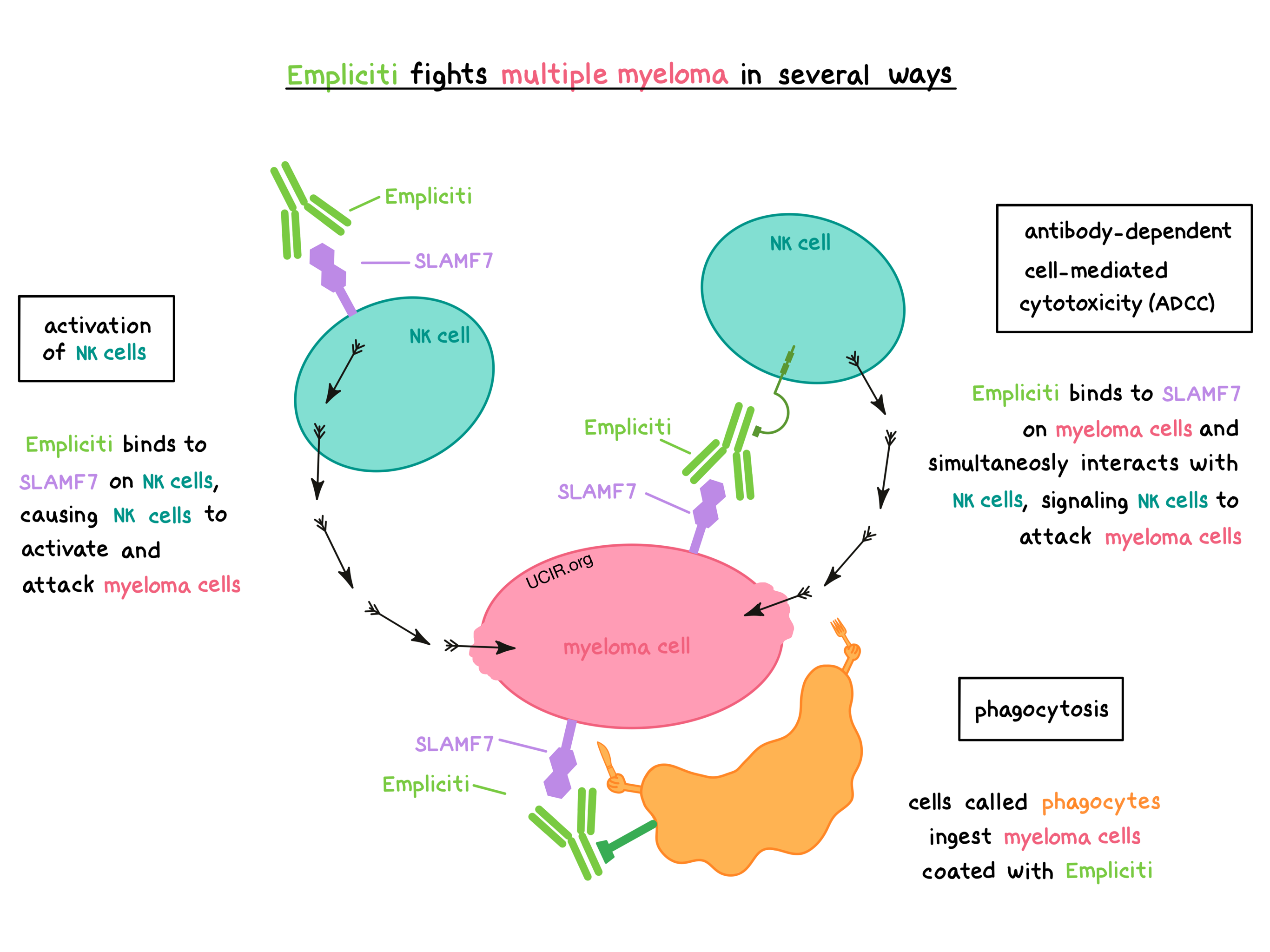
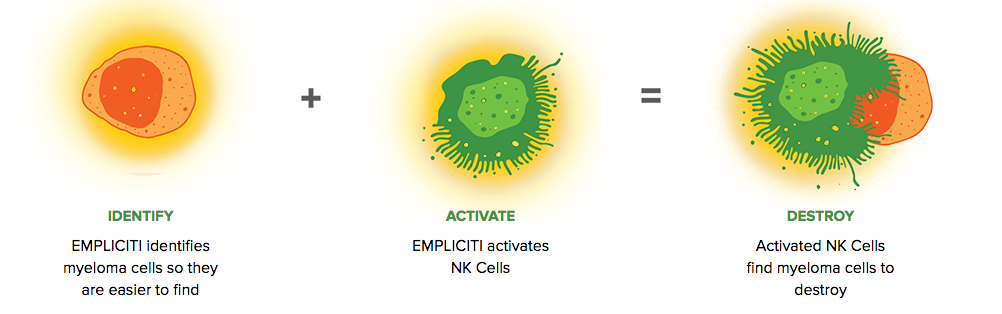
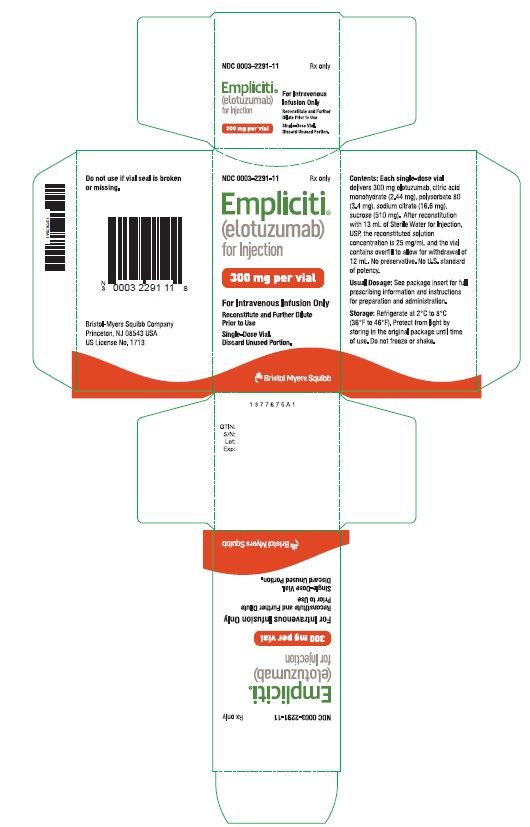
Bristol-Myers Squibb and AbbVie Receive FDA Approval of Empliciti™ (elotuzumab) for the Treatment of Patients with Multiple Myeloma Who Have Received One to Three Prior Therapies